What Is Sodium Sulphate?
Sodium sulphate (Na2SO4)
is a chemical compound that can be found as a mineral in nature or
derived from certain industrial processes as a by product. Sodium sulphate is commonly used to make soaps, detergents, pulp industry, textile industry other chemicals industry.
| PRODUCT IDENTIFICATION | ||
| CAS NO. | 7757-82-6 (Anhydrous)7727-73-3 (Decahydrate) | 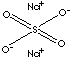 |
| EINECS NO. | 231-820-9 | |
| FORMULA | Na2SO4 | |
| MOL WT. | 142.04 | |
| H.S. CODE | 2833.11.5010 | |
| TOXICITY | ||
| SYNONYMS | Disodium monosulfate; Sulfuric acid sodium salt; | |
| Disodium sulfate; Sodium sulfate; Sulfuric acid sodium salt; Sulfuric acid disodium salt; Sulfuric acid disodium salt; Salt cake; Bisodium sulfate; Sodium sulfate (2:1); Thenardite; Natriumsulfat; Trona; Dibasic sodium sulfate; Other RN: 1337-28-6 | ||
| PHYSICAL AND CHEMICAL PROPERTIES | ||
| PHYSICAL STATE |
Hygroscopic white powder,
Odorless
|
|
| MELTING POINT |
880
- 888 C
|
|
| BOILING POINT | 1100 C (Decomposes) | |
| SPECIFIC GRAVITY |
2.66
- 2.75
|
|
| SOLUBILITY IN WATER | Soluble | |
| pH |
Aqueous solution
is neutral
|
|
| VAPOR DENSITY | ||
| AUTOIGNITION | ||
| NFPA RATINGS | Health: 1; Flammability: 0; Reactivity: 0 | |
| REFRACTIVE INDEX | ||
| FLASH POINT | ||
| STABILITY | Stable under ordinary conditions | |
Sodium Sulphate Manufacturing Process
There are two processes in sodium sulfate production. 1. Chemical process Feed sodium chloride (≥97) and sulfuric acid (H2SO4 ≥98) into Mannheim furnace, and the reactant is sodium sulfate. The process is as following: 2NaCl + H2SO4 Na2SO4 + 2HCl ↑- Q Actually, the reaction is proceeded in two steps: NaCl + H2SO4 NaHSO4 + HCl↑ + Q(1) NaHSO4+ NaCl Na2SO4 + 2HCl↑ - Q(2) The first step is an exothermal reaction that can be carried out at lower temperature, NaHSO4
is form first. While the second reaction is a strong endothermic
reaction, the reaction can be carried out only at 500 °C to 600 °C. The
heat required by the reaction is supplied by burning of gas in the
burning chamber of the furnace. The gas is generated by a coal gas
generator. The temperature in the burning chamber should be maintained
around 1200 °C. 2. Extracted from Glauberite rocks Crushed Glauberite
rocks are fed into a tank, and then is extracted by water. Remove
impurities in the extract. The clean extract is evaporated and
centrifuged. Finally this wet sodium sulfate is dried to obtain product
sodium sulfate. The process is proceeded as following: Na2SO4.CaSO4 + 2H2O = Na2SO4 + CaSO4.2H2O
Sodium Sulphate Uses
Use in Soaps and Detergents
Although the need is slowly being reduced, a large amount of sodium sulfate has been used in powdered detergents as filler during the last 30 years. This took place because phosphates, which were traditionally used as fillers in powder detergents, were discovered to be detrimental to the environment. However, sodium sulfate use has begun declining as well, the need for filler has gone down, due to the trend toward using concentrated liquid detergents instead of bulkier powder formulas. It is still used in carpet powders and window defrosting applications.
Textiles
Approximately 100,000 tons of sodium sulfate are utilized annually in Japan and the U.S. for dyeing textiles. It is an ideal compound for this purpose, because it does not corrode the stainless steel vessels as sodium chloride (which can also be used in this manner) does. Sodium sulfate is a leveling agent, reducing negative chargers on the fibers, which allows the dyes to penetrate evenly. Sodium sulfate is a by-product of rayon production, and when there was a decreased need for Japanese rayon, the U.S. was able to fulfill the need for the compound that Japan had previously supplied.
Wood Pulp
One notable use for sodium sulfate compound is in the Kraft process, also known as the sulfate process, of wood pulp manufacturing which is widely used to make paper products and building supplies. Although other processes are now used, the Kraft process has been the dominant method of wood pulping since the 1940s.The technology involves impregnating wood chips with sodium sulfate; the wood is heated, causing a reduction of the sodium sulfate into sodium sulfide. This breaks the bond in the cellulose of the wood, making it malleable and able to be extruded.
Glass
Sodium sulfate is used in the glass industry as well. European glassmakers consume a significant amount of sodium sulfate per year, using up to approximately 110,000 tons per annum. The U.S. utilizes about 30,000 tons in glass making, as well. Sodium sulfate prevents scum formation by the molten glass during refining, and also fluxes the glass. The compound also acts as a fining agent in molten glass, removing small air bubbles and imperfections during the blowing and casting processes.
Drying and Thermal Storage
In the laboratory, sodium sulfate is often used as an inert drying compound for organic materials. It removes water from compounds reliably at temperatures below 30° C (86° F). Another main use of sodium sulfate is in thermal storage. It has been utilized as a solar heat storage component since the 1950s, because it has a high heat storage capacity and does not change from a solid to a liquid until 90 ° F (32 ° C). Sodium sulfate is used to store heat in thermal tiles, and put into cells surrounded by solar-heated water, as well as in some computer-cooling and insulating applications.
Sodium sulfate is still widely in use today, and should continue to be so for some time. Even though some manufacturing processes are phasing out use of the compound, other purposes are continuing to be found. Sodium sulfate’s unique heat storage properties make it an ideal candidate for use in many future processes and products.
Although the need is slowly being reduced, a large amount of sodium sulfate has been used in powdered detergents as filler during the last 30 years. This took place because phosphates, which were traditionally used as fillers in powder detergents, were discovered to be detrimental to the environment. However, sodium sulfate use has begun declining as well, the need for filler has gone down, due to the trend toward using concentrated liquid detergents instead of bulkier powder formulas. It is still used in carpet powders and window defrosting applications.
Textiles
Approximately 100,000 tons of sodium sulfate are utilized annually in Japan and the U.S. for dyeing textiles. It is an ideal compound for this purpose, because it does not corrode the stainless steel vessels as sodium chloride (which can also be used in this manner) does. Sodium sulfate is a leveling agent, reducing negative chargers on the fibers, which allows the dyes to penetrate evenly. Sodium sulfate is a by-product of rayon production, and when there was a decreased need for Japanese rayon, the U.S. was able to fulfill the need for the compound that Japan had previously supplied.
Wood Pulp
One notable use for sodium sulfate compound is in the Kraft process, also known as the sulfate process, of wood pulp manufacturing which is widely used to make paper products and building supplies. Although other processes are now used, the Kraft process has been the dominant method of wood pulping since the 1940s.The technology involves impregnating wood chips with sodium sulfate; the wood is heated, causing a reduction of the sodium sulfate into sodium sulfide. This breaks the bond in the cellulose of the wood, making it malleable and able to be extruded.
Glass
Sodium sulfate is used in the glass industry as well. European glassmakers consume a significant amount of sodium sulfate per year, using up to approximately 110,000 tons per annum. The U.S. utilizes about 30,000 tons in glass making, as well. Sodium sulfate prevents scum formation by the molten glass during refining, and also fluxes the glass. The compound also acts as a fining agent in molten glass, removing small air bubbles and imperfections during the blowing and casting processes.
Drying and Thermal Storage
In the laboratory, sodium sulfate is often used as an inert drying compound for organic materials. It removes water from compounds reliably at temperatures below 30° C (86° F). Another main use of sodium sulfate is in thermal storage. It has been utilized as a solar heat storage component since the 1950s, because it has a high heat storage capacity and does not change from a solid to a liquid until 90 ° F (32 ° C). Sodium sulfate is used to store heat in thermal tiles, and put into cells surrounded by solar-heated water, as well as in some computer-cooling and insulating applications.
Sodium sulfate is still widely in use today, and should continue to be so for some time. Even though some manufacturing processes are phasing out use of the compound, other purposes are continuing to be found. Sodium sulfate’s unique heat storage properties make it an ideal candidate for use in many future processes and products.



1 comments:
commentsI am grateful for this blog to distribute knowledge about this significant topic. Here I found different segments and now I am going to use these new instructions with new enthusiasm.รับผลิตสบู่
Reply